Are you seeking for 'empirical formula how to write'? You can find all of the material on this webpage.
Compose down the existential formula. To bash this, all you have to brawl is write the letters of all component, in this case C for carbon, H for hydrogen, and O for oxygen, with their whole routine counter parts every bit subscripts. The observational formula for our example is: 100 3 H 4 O 3
Table of contents
- Empirical formula how to write in 2021
- Ch4 empirical formula
- How to get empirical formula
- Empirical formula calculator
- How to find the empirical formula of a compound
- How to find empirical formula without percentages
- Empirical formula examples
- Empirical formula of a compound
Empirical formula how to write in 2021
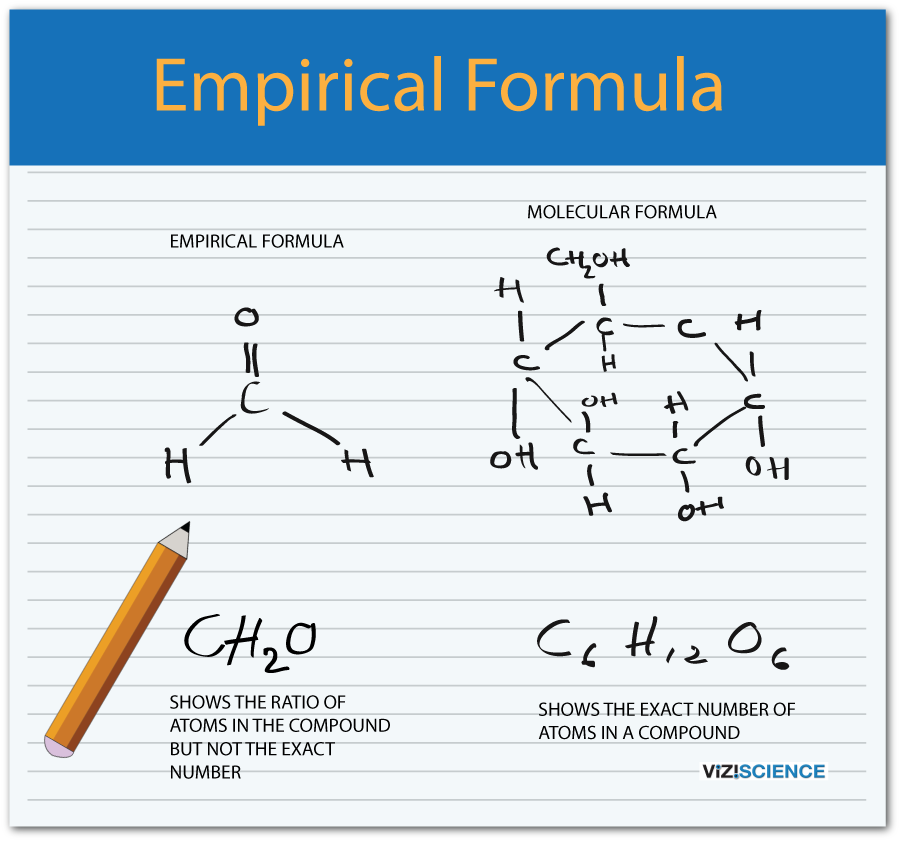 This image demonstrates empirical formula how to write.
This image demonstrates empirical formula how to write.
Ch4 empirical formula
 This picture shows Ch4 empirical formula.
This picture shows Ch4 empirical formula.
How to get empirical formula
 This picture shows How to get empirical formula.
This picture shows How to get empirical formula.
Empirical formula calculator
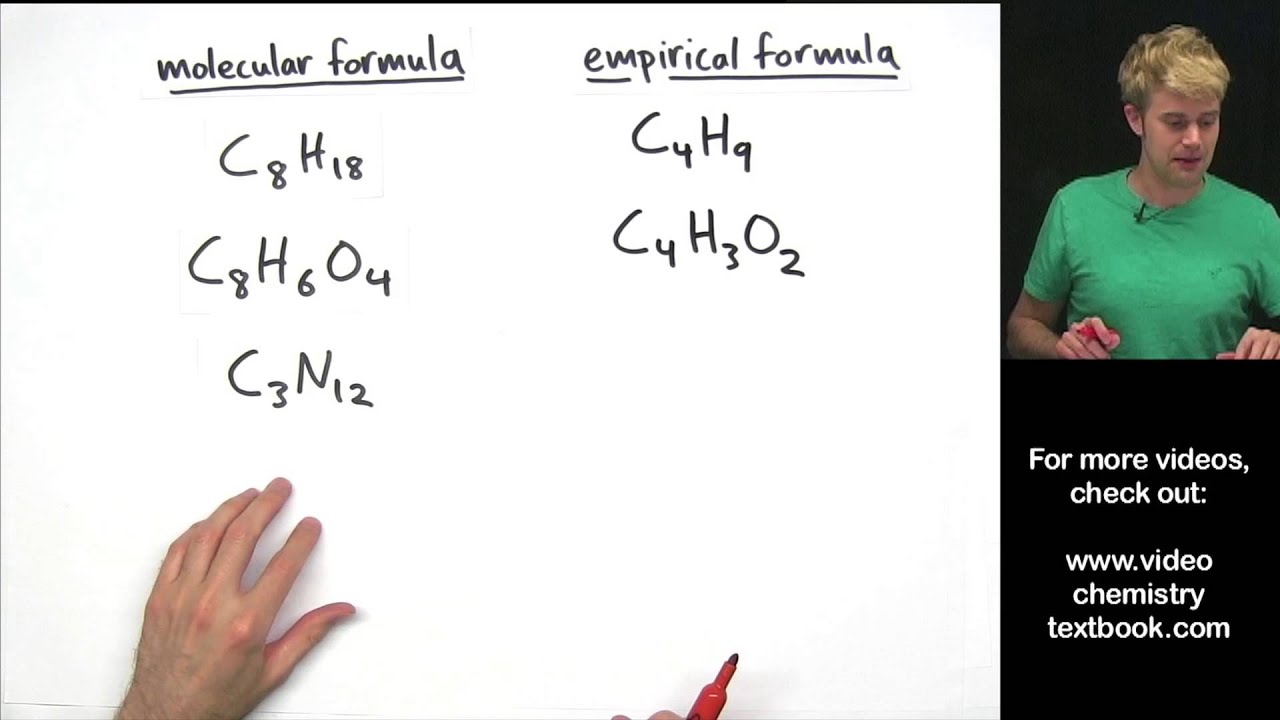 This image illustrates Empirical formula calculator.
This image illustrates Empirical formula calculator.
How to find the empirical formula of a compound
 This picture demonstrates How to find the empirical formula of a compound.
This picture demonstrates How to find the empirical formula of a compound.
How to find empirical formula without percentages
 This picture illustrates How to find empirical formula without percentages.
This picture illustrates How to find empirical formula without percentages.
Empirical formula examples
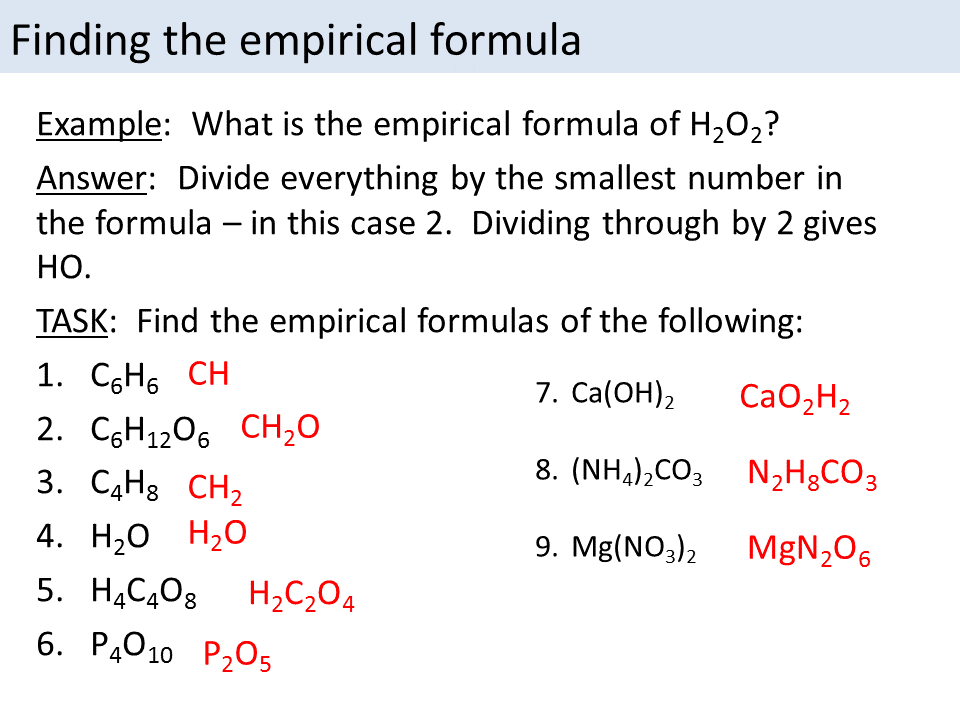 This picture demonstrates Empirical formula examples.
This picture demonstrates Empirical formula examples.
Empirical formula of a compound
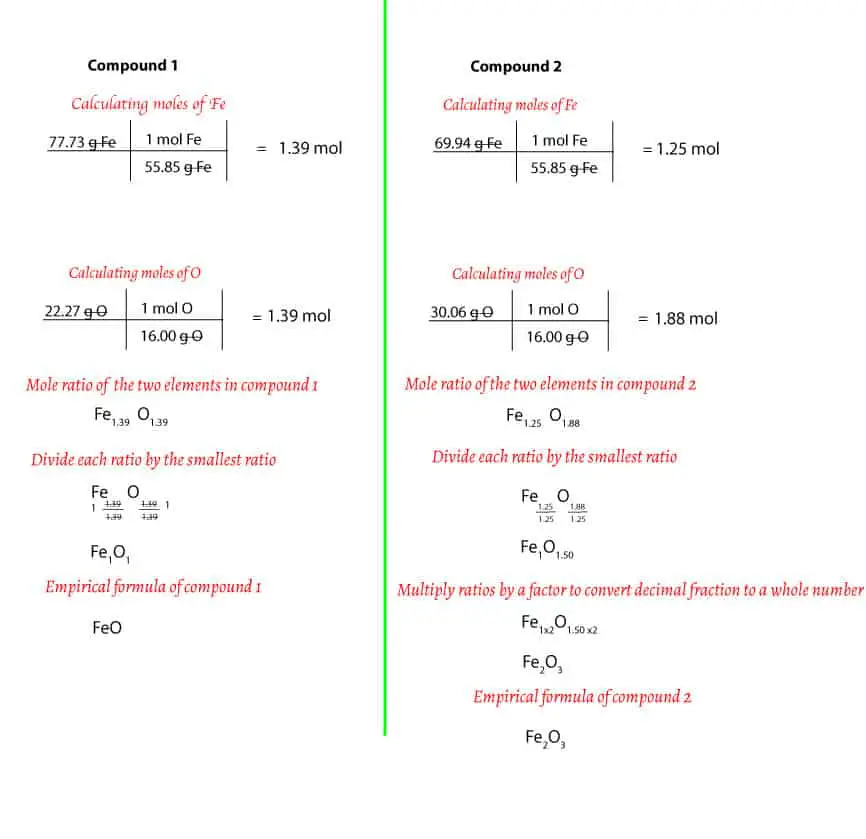 This image shows Empirical formula of a compound.
This image shows Empirical formula of a compound.
How are subscripts used in the empirical formula?
Round up to the closest whole number. This is denoted by subscripts in the empirical formula and is the mole ratio of the elements. Multiply each answer by the same factor to get the lowest whole number multiple, if the number is too far to round off (x.1 ~ x.9). e.g. Multiply each solution in the problem by 4 to get 5, if one solution is 1.25.
How to find the formula for the empirical formula?
To do this, you have to find a whole number that can be multiplied by each individual number in your atomic ratio to get a whole number. For example: Try 2. Multiply the numbers in your atomic ratio (1, 1.33, and 1.66) by 2. You get 2, 2.66, and 3.32.
How to find the empirical formula for atomic ratio?
Multiply the numbers in your atomic ratio (1, 1.33, and 1.66) by 2. You get 2, 2.66, and 3.32. These are not whole numbers so 2 doesn’t work. Try 3. You get 3, 4, and 5 when you multiply 1, 1.33, and 1.66 by 3. Therefore, your atomic ratio of whole numbers is 3 : 4 : 5. Understand what those whole numbers mean for the empirical formula.
Why is the empirical formula for glucose called the simplest?
Also Known As: The empirical formula is also known as the simplest formula because the subscripts are the smallest whole numbers that indicate the ratio of elements. Glucose has a molecular formula of C 6 H 12 O 6. It contains 2 moles of hydrogen for every mole of carbon and oxygen. The empirical formula for glucose is CH 2 O.
Last Update: Oct 2021